
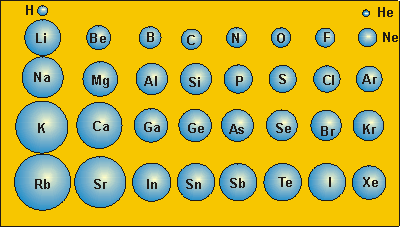
The shade of the box ranges from red to yellow as the radius increases gray indicates lack of data.Įxplanation of the general trends Ī graph comparing the atomic radius of elements with atomic numbers 1–100. The values are in picometers (pm or 1×10 −12 m), with an accuracy of about 5 pm. The following table shows empirically measured covalent radii for the elements, as published by J. Although the model itself is now obsolete, the Bohr radius for the hydrogen atom is still regarded as an important physical constant.Įmpirically measured atomic radius It is only applicable to atoms and ions with a single electron, such as hydrogen, singly ionized helium, and positronium. Bohr radius: the radius of the lowest-energy electron orbit predicted by Bohr model of the atom (1913).Metallic radius: the nominal radius of atoms of an element when joined to other atoms by metallic bonds.In principle, the distance between two atoms that are bound to each other in a molecule (the length of that covalent bond) should equal the sum of their covalent radii. Covalent radius: the nominal radius of the atoms of an element when covalently bound to other atoms, as deduced from the separation between the atomic nuclei in molecules.In principle, the spacing between two adjacent oppositely charged ions (the length of the ionic bond between them) should equal the sum of their ionic radii. Ionic radius: the nominal radius of the ions of an element in a specific ionization state, deduced from the spacing of atomic nuclei in crystalline salts that include that ion.Because Van der Waals interactions arise through quantum fluctuations of the atomic polarisation, the polarisability (which can usually be measured or calculated more easily) may be used to define the Van der Waals radius indirectly.
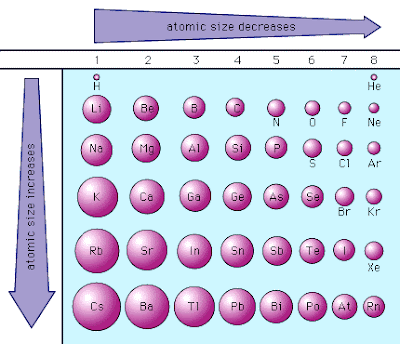
The Van der Waals radius may be defined even for elements (such as metals) in which Van der Waals forces are dominated by other interactions. Van der Waals radius: In the simplest definition, half the minimum distance between the nuclei of two atoms of the element that are not otherwise bound by covalent or metallic interactions.Widely used definitions of atomic radius include: However, in 1923, when more crystal data had become available, it was found that the approximation of an atom as a sphere does not necessarily hold when comparing the same atom in different crystal structures. In 1920, shortly after it had become possible to determine the sizes of atoms using X-ray crystallography, it was suggested that all atoms of the same element have the same radii.

Therefore, the radius of an atom is more than 10,000 times the radius of its nucleus (1–10 fm), and less than 1/1000 of the wavelength of visible light (400–700 nm). Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm ( trillionths of a meter), or between 0.3 and 3 ångströms. Moreover, in condensed matter and molecules, the electron clouds of the atoms usually overlap to some extent, and some of the electrons may roam over a large region encompassing two or more atoms. Rather, their positions must be described as probability distributions that taper off gradually as one moves away from the nucleus, without a sharp cutoff these are referred to as atomic orbitals or electron clouds. Įlectrons do not have definite orbits nor sharply defined ranges. The value of the radius may depend on the atom's state and context.
The dependencies on environment, probe, and state lead to a multiplicity of definitions.ĭepending on the definition, the term may apply to atoms in condensed matter, covalently bonding in molecules, or in ionized and excited states and its value may be obtained through experimental measurements, or computed from theoretical models. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state however theoretical calculations are simpler when considering atoms in isolation. Four widely used definitions of atomic radius are: Van der Waals radius, ionic radius, metallic radius and covalent radius. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Diagram of a helium atom, showing the electron probability density as shades of gray.


 0 kommentar(er)
0 kommentar(er)
